
The process of efficient separation and purification in laboratory settings relies on a variety of intricate instruments. These devices enable scientists and researchers to achieve precise results in their experiments and analyses. To fully grasp how these systems function, it is crucial to explore the individual elements that contribute to their overall operation.
By delving into the specific components, one can gain insight into how each part plays a pivotal role in facilitating the distillation process. Understanding the interactions between these elements not only enhances operational efficiency but also aids in troubleshooting and optimizing performance.
In this section, we will outline the various components of these sophisticated systems, highlighting their functions and significance. By familiarizing ourselves with these essential items, we can better appreciate the complexities involved in achieving successful separations in laboratory applications.
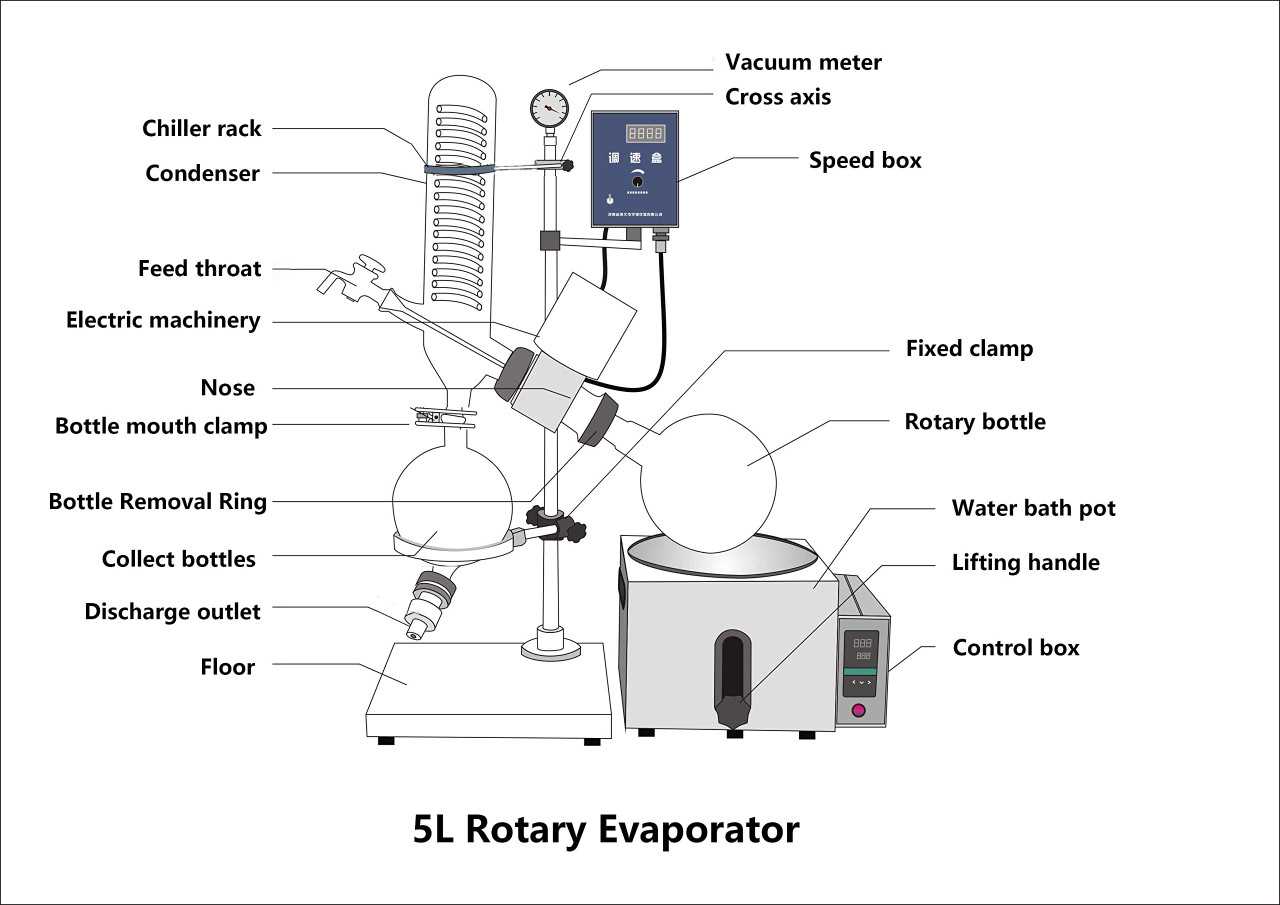
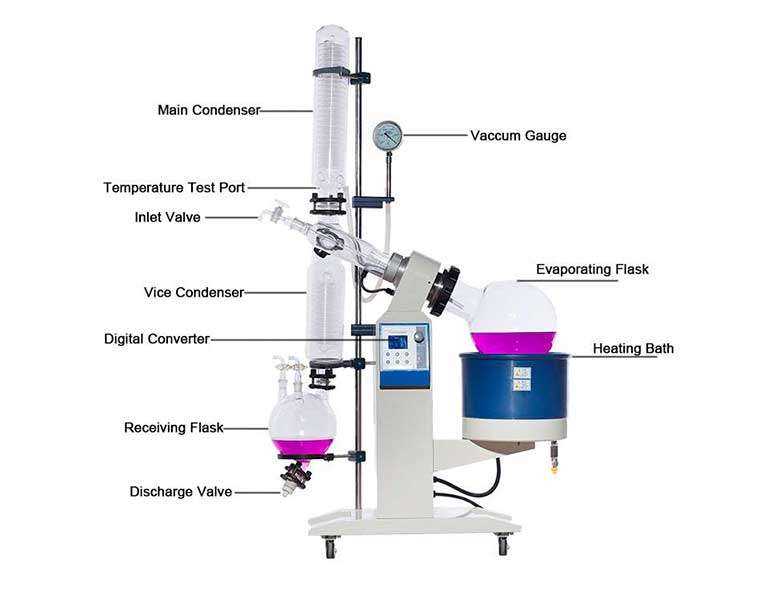
Understanding the essential elements of this distillation apparatus is crucial for effective operation and maintenance. Each component plays a significant role in ensuring optimal performance during the evaporation process.
- Heating Bath: Provides consistent warmth to facilitate the evaporation of solvents.
- Evaporating Flask: Holds the liquid mixture that needs to be distilled.
- Condenser: Cools and condenses vapor back into liquid form for collection.
- Receiving Flask: Collects the condensed liquid after evaporation.
- Vacuum System: Reduces pressure, lowering the boiling point of the solvent for efficient evaporation.
Each of these components contributes to the overall efficiency and effectiveness of the system, making it vital for users to understand their functions and interconnections.
Importance of Each Part
The functionality of any complex apparatus relies heavily on the individual components that contribute to its overall performance. Understanding the significance of each segment is essential for effective operation and maintenance. Each element serves a unique role, working in harmony with others to achieve the desired outcomes.
The heating element is crucial for initiating the process by providing the necessary temperature control, ensuring efficient evaporation. Meanwhile, the condenser plays an equally important role, facilitating the cooling of vapor back into liquid form, thus optimizing recovery. Additionally, the receiving flask is vital for collecting the distilled liquid, while the vacuum system enhances the efficiency by reducing pressure, allowing for lower boiling points and preventing thermal degradation of sensitive substances.
Each segment not only contributes to the machine’s efficiency but also ensures the safety and reliability of the entire system. By appreciating the specific functions of these components, users can better troubleshoot issues and enhance their overall experience with the equipment.
Operational Principles of Rotovaps
The mechanism behind these essential laboratory instruments revolves around the effective removal of solvents through a combination of heat and reduced pressure. By creating a vacuum environment, the boiling point of liquids is lowered, allowing for efficient evaporation at lower temperatures. This process is particularly beneficial for heat-sensitive compounds that could degrade if exposed to elevated temperatures.
The system typically involves a rotating flask that increases the surface area of the liquid, enhancing the evaporation rate. As the flask spins, the solvent forms a thin film along the walls, further facilitating its transition to vapor. This vapor is then condensed back into liquid form, usually in a separate cooling chamber, allowing for easy collection and recycling.
By optimizing these operational parameters, users can achieve high levels of efficiency while minimizing the risk of thermal degradation of their samples. The careful control of temperature, rotation speed, and pressure settings ensures that the entire process is both effective and gentle on the materials being processed.
Types of Rotovap Systems
Various systems are designed to facilitate the efficient separation of solvents from samples, each tailored to specific applications and user needs. Understanding these different configurations can significantly enhance the process and outcomes of sample preparation in laboratory settings.
Classic Configurations
The traditional setups consist of a boiling flask, a condenser, and a collection vessel. These systems rely on heat and vacuum to lower boiling points, allowing for effective solvent removal while preserving the integrity of the materials. Key advantages include simplicity and effectiveness, making them a popular choice for many laboratories.
Advanced Variants
Modern iterations incorporate automated features and enhanced control mechanisms, offering improved precision and efficiency. Systems may include digital monitoring and adjustable parameters for temperature and pressure, which can greatly optimize the separation process. These advancements make them suitable for more complex applications in research and industrial environments.
Choosing the Right Configuration
Selecting the optimal setup for your laboratory equipment is crucial for achieving efficient results. The configuration impacts not only the effectiveness of your processes but also the ease of operation and safety. Understanding the different components and their functions can guide you in making informed choices that best suit your needs.
Here are key considerations to keep in mind when determining the best arrangement:
- Purpose of Use: Define what you intend to achieve. Different setups cater to specific applications, such as distillation or solvent removal.
- Space Availability: Assess the physical space in your lab. Ensure that the chosen configuration fits comfortably without obstructing workflow.
- Temperature Control: Consider how temperature regulation will be managed. Some setups allow for more precise control, which can be vital for sensitive processes.
- Vacuum Requirements: Identify the level of vacuum needed for your operations. Certain configurations may offer better vacuum performance than others.
- Ease of Maintenance: Choose a design that facilitates easy cleaning and maintenance, minimizing downtime and maximizing productivity.
By carefully evaluating these factors, you can select a configuration that enhances performance and aligns with your laboratory objectives.
Maintenance Tips for Rotovap Parts

Proper upkeep of your apparatus is crucial for ensuring optimal performance and longevity. Regular maintenance not only enhances efficiency but also prevents potential issues that could arise from neglect.
Routine Cleaning
- Always clean glass components with appropriate solvents to avoid residue buildup.
- Inspect seals and gaskets regularly for signs of wear and replace them as needed.
- Wipe down exterior surfaces to prevent contamination and maintain a tidy workspace.
Periodic Inspections
- Check all connections and fittings to ensure they are secure and functioning correctly.
- Examine the heating element for any signs of damage or malfunction.
- Monitor the vacuum system for leaks or performance drops, addressing issues promptly.
Common Issues and Solutions
This section addresses frequent challenges encountered during the operation of distillation equipment and offers effective strategies for resolution. Understanding these common problems can enhance efficiency and prevent potential disruptions.
Frequent Challenges
- Poor vacuum levels
- Temperature inconsistencies
- Leaks in the system
- Loss of solvent
Effective Solutions
- Check seals and connections to ensure a tight fit.
- Regularly calibrate temperature settings for accurate results.
- Inspect vacuum lines for blockages or damage.
- Utilize solvent recovery systems to minimize waste.
Safety Considerations When Using Rotovaps
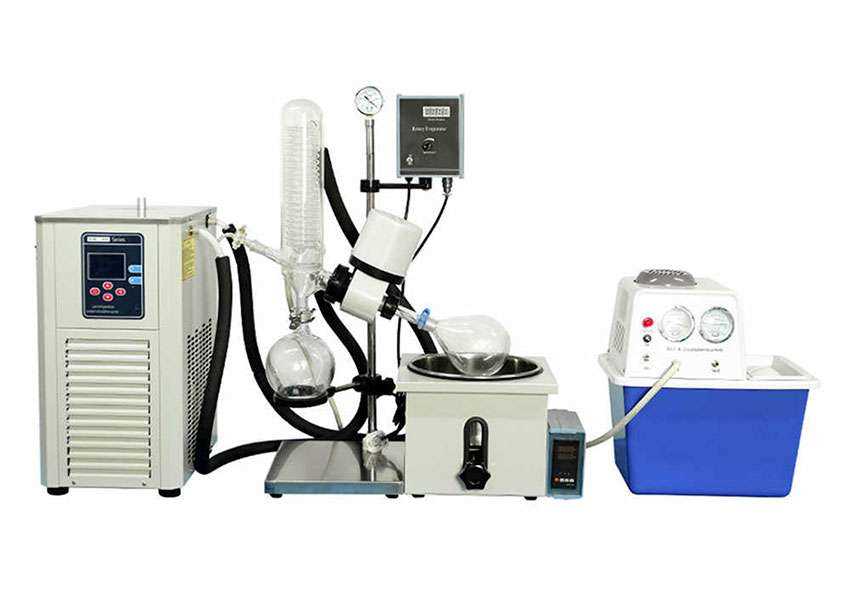
Ensuring a secure working environment is crucial when operating distillation apparatus. Proper safety measures help prevent accidents and protect both personnel and equipment during the process.
Always wear appropriate personal protective equipment, including gloves, goggles, and lab coats, to safeguard against potential hazards. Ensure that the workspace is well-ventilated to minimize the risk of inhaling harmful vapors. Regularly inspect the equipment for any signs of wear or damage, as this can lead to malfunctions.
Familiarize yourself with the operational manual and emergency procedures before starting any distillation. Avoid overfilling the flask to prevent spills and ensure that all connections are secure. It is also advisable to use a water bath at a controlled temperature to avoid overheating.
Lastly, maintain a clean and organized workspace. Promptly clean up any spills and properly dispose of waste materials to minimize risks. Adhering to these guidelines will significantly enhance safety during the operation of this essential laboratory equipment.
Upgrading Rotovap Components
Enhancing the efficiency and functionality of a distillation apparatus can significantly improve experimental outcomes. By selecting high-quality components and implementing advanced technologies, users can optimize performance, reduce processing time, and increase overall productivity.
Key Components for Upgrade
Several critical elements contribute to the system’s effectiveness. Upgrading these components can yield notable benefits:
| Component | Benefits of Upgrading |
|---|---|
| Condenser | Improved cooling efficiency and reduced vapor loss. |
| Vacuum Pump | Enhanced pressure control for faster distillation rates. |
| Heating Mantle | More precise temperature control for better solvent recovery. |
Considerations for Component Selection
When selecting new components, factors such as compatibility, material quality, and performance specifications should be considered. Investing in superior elements not only enhances the apparatus but also ensures longevity and reliability in various applications.
Applications in Laboratory Settings
In laboratory environments, specialized equipment plays a crucial role in various processes, facilitating efficient and effective research. This technology is particularly valuable for tasks involving the separation and purification of compounds, where precise control over temperature and pressure is essential.
Common Uses
- Solvent removal: Efficiently evaporating solvents from mixtures, allowing for the recovery of valuable compounds.
- Concentration of samples: Reducing the volume of solutions for further analysis, enhancing the concentration of target substances.
- Purification: Isolating specific components from complex mixtures, ensuring higher purity levels in the final product.
Benefits in Research
- Time efficiency: Accelerating processes that would otherwise take significantly longer, thereby increasing overall productivity.
- Enhanced safety: Minimizing exposure to hazardous materials through closed systems, reducing risks associated with volatile solvents.
- Improved reproducibility: Standardizing conditions to yield consistent results across different experiments, fostering reliable data collection.
Understanding Distillation Processes
Distillation is a widely used technique that involves separating components of a mixture based on differences in their boiling points. This process is essential in various fields, including chemistry, pharmaceuticals, and food production, as it allows for the purification and concentration of desired substances.
At its core, the method relies on heating a liquid to create vapor, which is then cooled and condensed back into a liquid. This cycle can be repeated multiple times to achieve higher purity levels. Factors such as temperature control, pressure, and the nature of the substances involved play a crucial role in the efficiency and effectiveness of the separation.
In practice, understanding the principles behind this technique enables researchers and professionals to optimize their processes for better yields and higher quality outputs. The ability to manipulate various parameters enhances the adaptability of distillation methods to different applications, making it a fundamental skill in scientific and industrial environments.