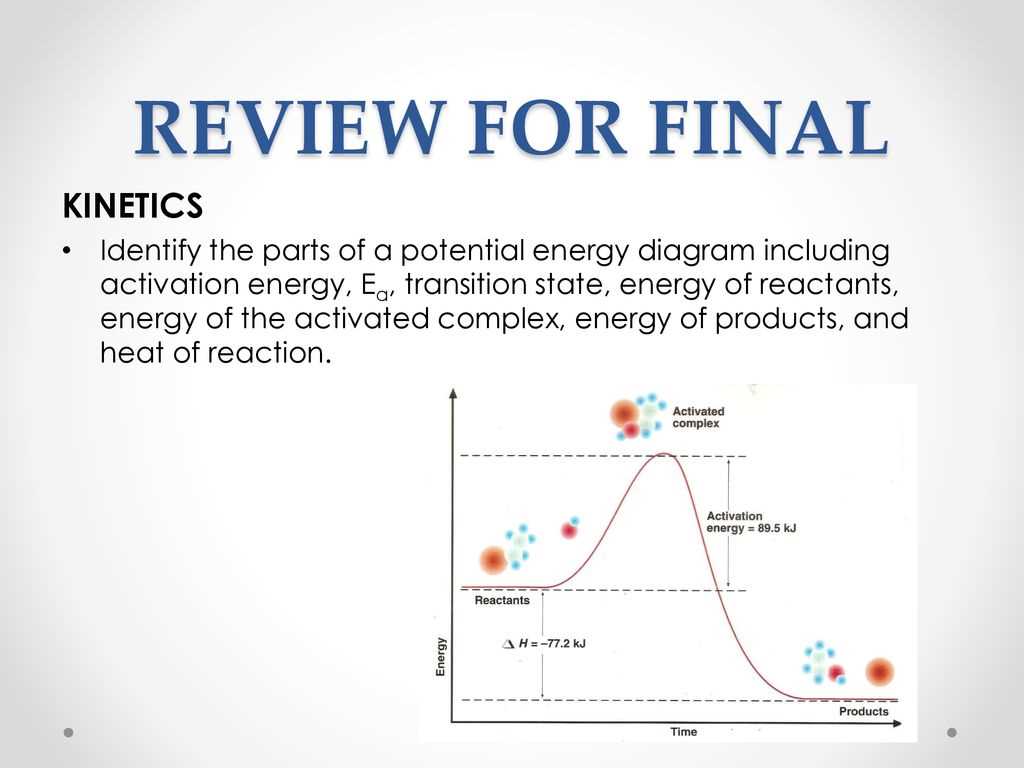
In the study of various physical phenomena, graphical representations play a crucial role in conveying complex concepts. These visual tools allow for the analysis of systems and processes by illustrating the relationships between different variables. A specific type of representation focuses on how certain quantities change, revealing valuable insights about the behavior of a system under various conditions.
Within this context, several key features emerge that provide a deeper understanding of the underlying principles. Each element of the graphical representation contributes to a comprehensive view of the interactions at play. By examining these components, one can uncover the dynamics of the system and how it responds to different influences, ultimately enhancing our grasp of the scientific concepts involved.
Furthermore, recognizing the significance of each aspect within the visual framework enables clearer communication of ideas. This understanding is essential for students and professionals alike, as it facilitates a more nuanced discussion of the principles governing physical systems. By exploring the elements involved, one can appreciate the complexity and elegance of the phenomena being studied.
This section aims to explore the various components that make up a graphical representation of the stored energy within a system. By understanding these elements, readers can gain insights into the behaviors and transitions that occur during different processes.
Key elements to consider include:
- Curves: The lines that indicate changes in stored energy levels.
- Peaks: The highest points representing maximum stored energy under specific conditions.
- Valleys: The lowest points indicating minimal stored energy, often associated with stable configurations.
- Horizontal Sections: Areas where the stored energy remains constant, suggesting equilibrium.
- Transitions: Regions where the energy shifts from one level to another, often indicating a change in state.
Understanding these elements helps to visualize how energy is transformed and how systems respond to various influences. This knowledge is crucial for analyzing reactions and mechanisms in physical and chemical processes.
Key Components of Energy Diagrams
This section explores essential elements that contribute to the understanding of graphical representations used to illustrate changes in a system’s state. These components are crucial for interpreting the transitions and interactions that occur within various processes.
Major Elements
- Axes: The horizontal and vertical lines that establish the framework for plotting values.
- Curves: Lines that indicate the relationship between different states or conditions.
- Reference Points: Specific locations that serve as benchmarks for comparison.
Transitional Features
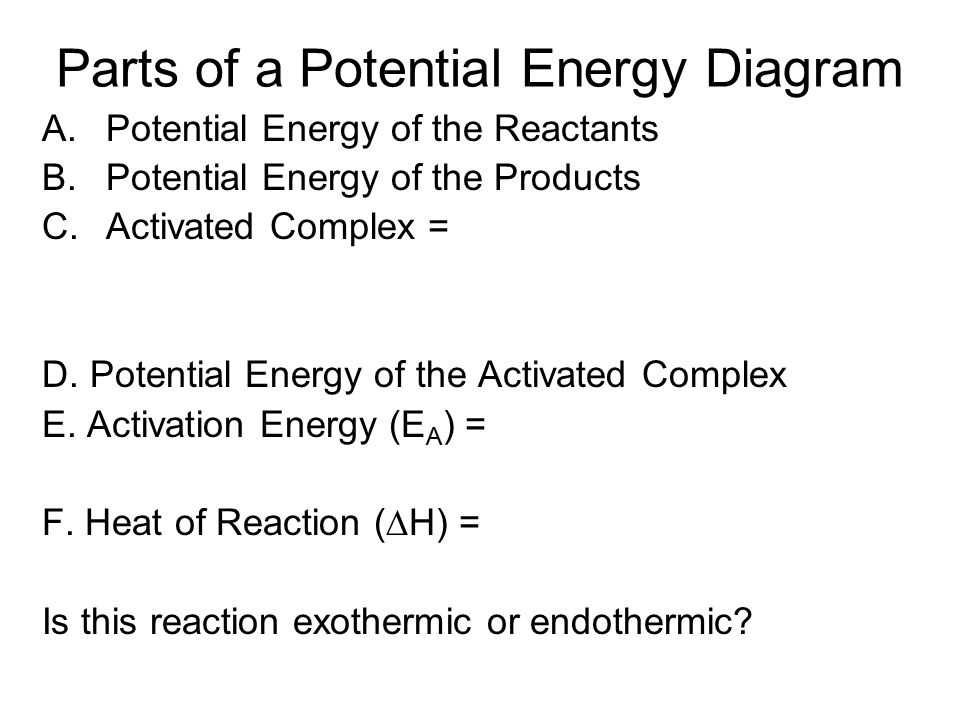
- Peaks: High points that suggest stability or potential for transformation.
- Valleys: Low points representing moments of transition or energy release.
- Plateaus: Flat regions indicating periods of equilibrium or consistency.
Types of Potential Energy Representations
The various forms of illustrating stored force can provide valuable insights into the behavior of systems. These visual tools serve to enhance understanding by depicting how the stored force changes under different conditions, allowing for a clearer analysis of the interactions involved.
Graphical Representations
One common approach is through graphical representations that showcase the relationship between position and stored force. These visuals can highlight key features, such as equilibrium points and maximum stored force, facilitating a deeper comprehension of the system’s dynamics.
Mathematical Models
Another method involves the use of mathematical models that express the relationship through equations. This approach allows for precise calculations and predictions, offering a quantitative perspective that can be essential for rigorous analysis.
Role of Energy Levels Explained
The concept of varying states within a system is fundamental to understanding how substances behave under different conditions. Each level signifies a distinct arrangement that can influence transitions, interactions, and stability. By examining these levels, we gain insight into the underlying principles that govern various phenomena in physics and chemistry.
Understanding the Hierarchy
At the core of this discussion is the hierarchy of these states, which helps clarify how materials respond to external influences. Each state possesses unique characteristics that dictate how energy is absorbed or released. This hierarchy not only illustrates stability but also highlights potential changes that may occur when a system is disturbed.
Implications for Chemical Reactions
In the context of chemical processes, the arrangement of these states plays a crucial role in determining reaction pathways. As substances transition from one state to another, they can either absorb or emit energy, which in turn affects the overall dynamics of the reaction. Understanding this flow aids in predicting the behavior of different materials in various scenarios.
Interpreting Energy Barriers in Diagrams
Understanding the obstacles present in graphical representations of energetic systems is crucial for grasping the underlying processes. These barriers play a significant role in determining how substances transition between various states. By analyzing these graphical features, one can infer critical insights into the behavior of the system under consideration.
The height of a barrier typically reflects the amount of effort required for a transformation to occur. A lower barrier suggests that the process is more feasible, while a higher one indicates increased difficulty. Observing the shapes and positions of these barriers in a graphical context allows for predictions about reaction rates and stability of different states.
Additionally, the spacing between barriers can offer valuable information regarding the relative likelihood of different transitions. Closer barriers may suggest competing pathways, whereas wider gaps indicate that certain processes are less probable. By delving into these aspects, one can gain a more comprehensive understanding of the dynamics at play in the system.
Potential Wells and Their Significance
In the realm of physical sciences, certain formations play a crucial role in understanding how objects interact within a defined field. These structures can trap or contain entities, influencing their behavior and movement. By examining these formations, one can gain insights into the stability and dynamics of various systems.
Understanding the Role of Such Formations is essential in multiple fields, including chemistry and physics. They serve as foundational concepts that illustrate how substances can exist in various states of stability. These formations help to visualize and analyze the forces at play when entities transition between different states or conditions.
Applications of these concepts are vast, ranging from molecular interactions to the behavior of larger systems. They assist scientists in predicting how entities will react under different circumstances, thereby enhancing our knowledge of chemical reactions and physical phenomena. Ultimately, these structures are integral to the broader comprehension of the laws that govern the natural world.
Applications in Chemical Reactions
The graphical representation of energy changes during chemical processes provides valuable insights into the mechanisms of reactions. These illustrations highlight the relationships between reactants and products, allowing chemists to predict reaction behavior and outcomes.
One significant application of these visual tools is in understanding activation barriers. By identifying the energy required for a reaction to proceed, scientists can design catalysts that lower these barriers, enhancing reaction rates. Catalysts play a crucial role in various industrial processes, making them more efficient and cost-effective.
Additionally, analyzing these representations helps in examining exothermic and endothermic reactions. Understanding how energy is released or absorbed during these transformations allows for better control of reaction conditions, optimizing yield and safety. Thermodynamic principles derived from these models guide chemists in selecting appropriate conditions for their experiments.
Influence of External Forces
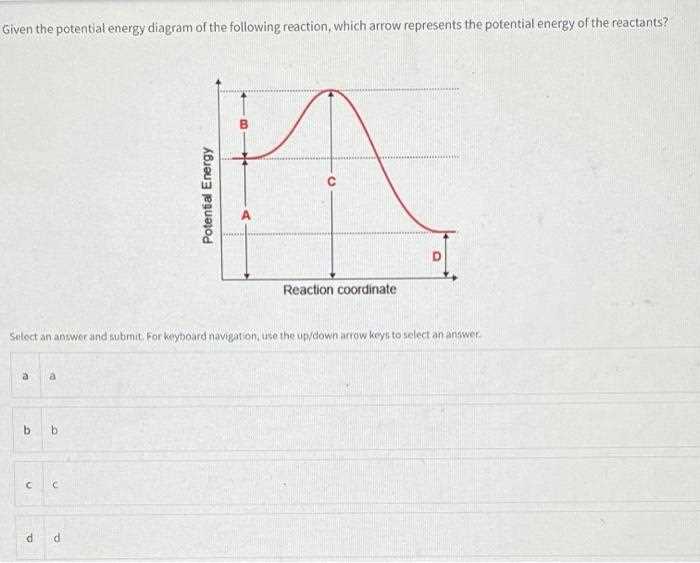
External factors play a significant role in shaping the behavior of a system. These influences can alter the path and characteristics of interactions, leading to varying outcomes in how a system evolves over time. Understanding these forces is crucial for comprehending how systems respond to different conditions.
Types of External Influences
- Gravitational Forces: These forces act on all objects with mass, impacting their movement and stability.
- Electromagnetic Forces: These interactions between charged particles can lead to changes in energy states.
- Frictional Forces: Resistance encountered when surfaces move against each other can slow down or alter motion.
Effects on System Behavior
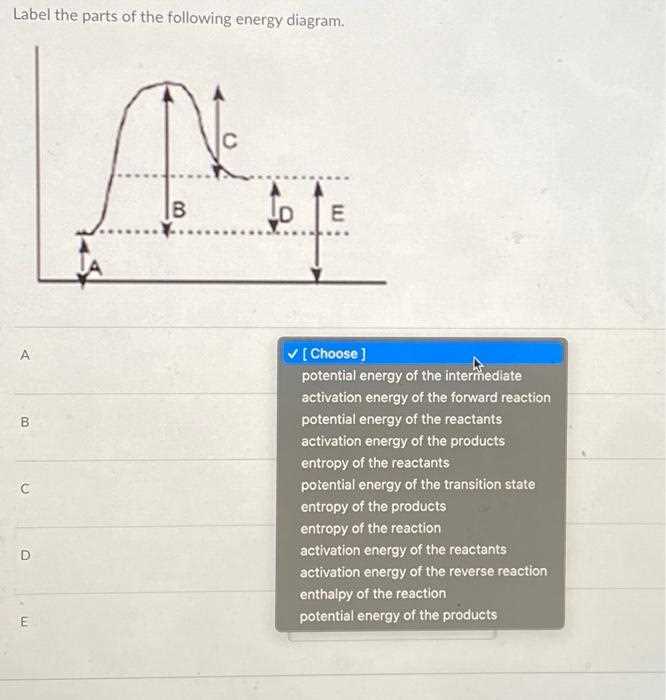
- Alteration of Pathways: External influences can change the trajectories of moving objects, affecting their final states.
- Energy Transfer: Forces can facilitate or hinder the transfer of motion and heat between entities.
- Stability of Equilibrium: External factors may shift the balance of a system, leading to new states of rest or activity.
Graphical Analysis of Stability Points
The graphical representation of a system’s behavior can reveal critical insights about its stability. By examining specific points on a graph, one can understand how a system responds to perturbations, which is essential for predicting its overall behavior.
In this analysis, we focus on various stability points, each of which plays a significant role in understanding the system’s dynamics. These points can be classified as:
- Stable Points: Locations where the system tends to return after a small disturbance.
- Unstable Points: Regions where any slight deviation leads the system further away from equilibrium.
- Saddle Points: Positions that exhibit characteristics of both stability and instability, often requiring careful consideration.
Identifying these points through graphical methods provides a visual context that enhances comprehension. The curvature of the graph around these points indicates how the system will behave under various conditions, leading to insights that can inform decision-making and design processes.
Overall, the graphical analysis serves as a powerful tool in evaluating the stability of a system, enabling a deeper understanding of its underlying mechanisms.
Transition States and Energy Changes
The concept of transition states plays a crucial role in understanding the transformations that occur during chemical reactions. These moments of change represent critical points where the reactants are in the process of being converted into products. Analyzing these stages provides insights into the variations in the system’s capacity to perform work throughout the reaction pathway.
Characteristics of Transition States
Transition states exhibit several distinctive features:
- They are temporary configurations that exist only for a brief period during the reaction.
- These states often have higher levels of internal energy compared to the initial reactants and final products.
- They can be influenced by various factors, including temperature and pressure, which can alter the speed and efficiency of reactions.
Implications of Energy Changes
Understanding the shifts in energy associated with transition states is essential for several reasons:
- It helps in predicting the rate of reactions, as higher energy barriers usually correspond to slower reactions.
- By identifying the energy levels involved, chemists can develop strategies to enhance reaction efficiency, such as using catalysts.
- Insights into these energy fluctuations can lead to advancements in various fields, including pharmaceuticals and materials science.
Identifying Equilibrium Positions
Understanding the concept of balance in various systems is crucial for analyzing their behavior. In many scenarios, certain points emerge where forces or influences acting on an object result in a state of stability. Recognizing these locations can provide insight into the system’s dynamics and predict its responses to external changes.
Characteristics of Equilibrium
Equilibrium points are characterized by specific conditions where the net force acting on an object is zero. At these junctures, small disturbances do not lead to significant changes in the system’s state. This section explores the traits that define these stable configurations, highlighting their significance in different contexts.
Examples of Equilibrium Positions
| System Type | Equilibrium Position | Description | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mechanical | Stable | Object returns to the original position after slight displacement. | ||||||||
| Chemical | Dynamic |
| Condition | Stored Energy | Moving Energy |
|---|---|---|
| At Rest | High | Low |
| In Motion | Low | High |
Real-World Examples of Energy Diagrams
This section explores various practical instances where graphical representations of forces and movements can be observed. These illustrations help in understanding how systems behave under different conditions and contribute to scientific analysis.
Examples in Everyday Life
Many situations in daily life can be illustrated through these visual tools:
- Roller Coasters: The rise and fall of a roller coaster can be analyzed using graphical representations, highlighting the conversion of kinetic and stored forms of force as the ride progresses.
- Falling Objects: When an object drops, the graphical representation shows the transition from stored to motion, emphasizing how gravitational influence affects the object’s speed.
- Stretching a Spring: Observing the force applied to stretch a spring demonstrates how energy shifts from one state to another, depicting potential and kinetic interactions.
Applications in Science and Engineering
In scientific and engineering contexts, these illustrations are essential for analysis:
- Chemical Reactions: In chemistry, graphical models illustrate the changes in states during reactions, showcasing how energy is absorbed or released.
- Mechanical Systems: Engineers utilize these visualizations to design machines, helping to predict performance by analyzing force distribution and movement.
- Renewable Energy Sources: Illustrations are employed to depict the transformation of natural resources into usable forms of power, such as in wind or solar technologies.